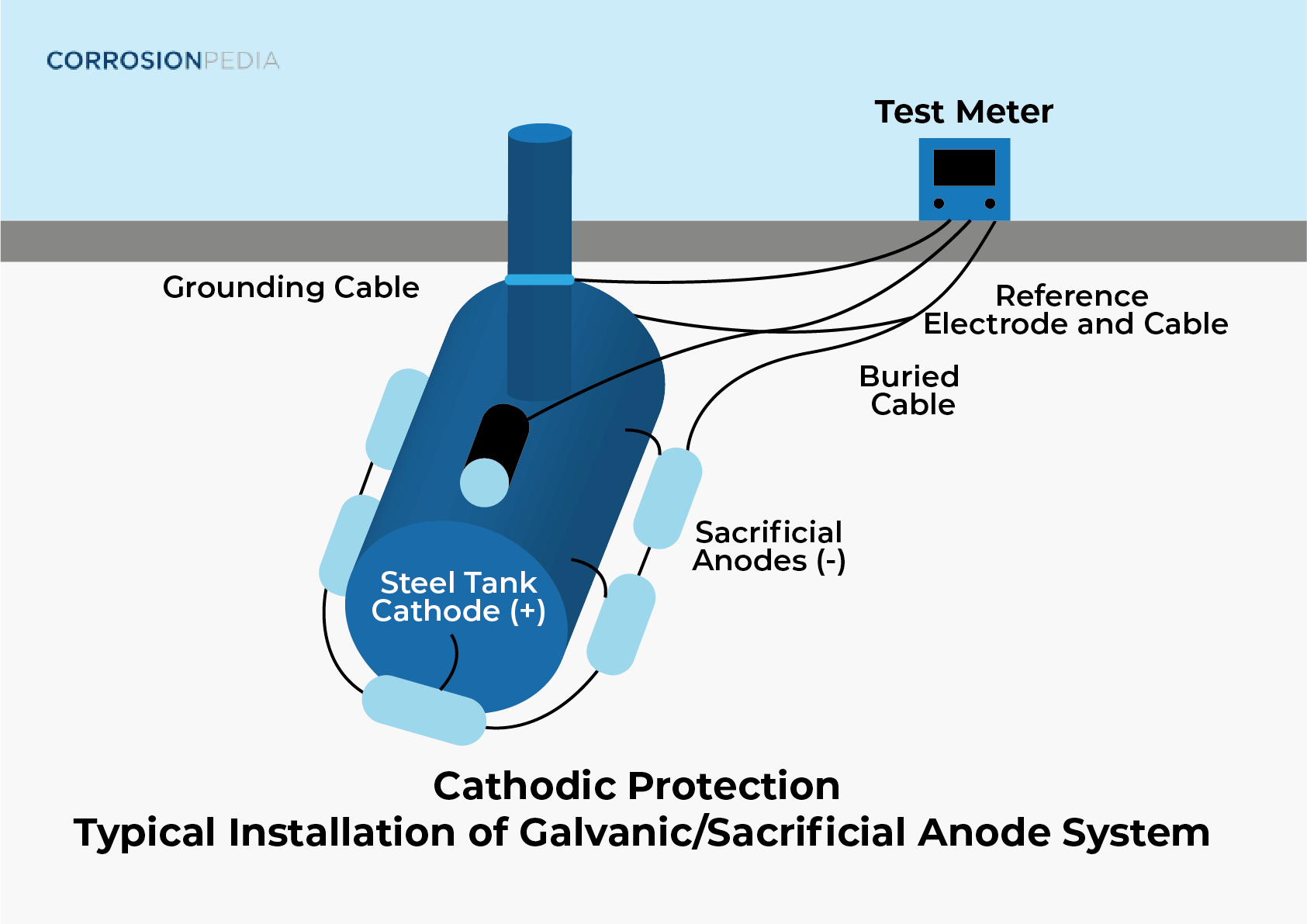
Why Coating Of Zinc On Iron Is Called Sacrificial Anode . According to the table of standard reduction potentials,. Sacrificial metals are widely used to prevent other metals from corroding: Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. Sacrificial coating is a type of metal coating which will undergo oxidation more than the metal surface that it protects. For example in galvanised steel. A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged. Magnesium has the most negative electropotential of the three. [3] many steel objects are coated with a. Sacrificial anodes generally come in three metals: Both zinc and aluminum produce potentials more negative. Because zinc is a more active metal than iron, it will act as the sacrificial anode in the electrochemical cell and dissolve (equation. The addition of zinc, a sacrificial anode, would prevent the iron metal from corroding. Sacrificial protection of iron using zinc is explained through a diagram of the electrochemical processes involved.
from www.corrosionpedia.com
[3] many steel objects are coated with a. Both zinc and aluminum produce potentials more negative. The addition of zinc, a sacrificial anode, would prevent the iron metal from corroding. According to the table of standard reduction potentials,. Magnesium has the most negative electropotential of the three. For example in galvanised steel. A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged. Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. Because zinc is a more active metal than iron, it will act as the sacrificial anode in the electrochemical cell and dissolve (equation. Sacrificial anodes generally come in three metals:
Galvanic Cathodic Protection
Why Coating Of Zinc On Iron Is Called Sacrificial Anode Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. For example in galvanised steel. Both zinc and aluminum produce potentials more negative. Sacrificial anodes generally come in three metals: Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. Because zinc is a more active metal than iron, it will act as the sacrificial anode in the electrochemical cell and dissolve (equation. Sacrificial coating is a type of metal coating which will undergo oxidation more than the metal surface that it protects. [3] many steel objects are coated with a. Sacrificial metals are widely used to prevent other metals from corroding: Magnesium has the most negative electropotential of the three. A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged. According to the table of standard reduction potentials,. The addition of zinc, a sacrificial anode, would prevent the iron metal from corroding. Sacrificial protection of iron using zinc is explained through a diagram of the electrochemical processes involved.
From www.youtube.com
Sacrificial Anode Cathodic Protection Allied Corrosion YouTube Why Coating Of Zinc On Iron Is Called Sacrificial Anode Magnesium has the most negative electropotential of the three. Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. Sacrificial protection of iron using zinc is explained through a diagram of the electrochemical processes involved. A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged. Sacrificial. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From www.propellerdepot.com
Why Zinc Anodes? Why Coating Of Zinc On Iron Is Called Sacrificial Anode Because zinc is a more active metal than iron, it will act as the sacrificial anode in the electrochemical cell and dissolve (equation. Sacrificial coating is a type of metal coating which will undergo oxidation more than the metal surface that it protects. A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From www.escpile.com
What is a Sacrificial Anode? Why Coating Of Zinc On Iron Is Called Sacrificial Anode For example in galvanised steel. Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. The addition of zinc, a sacrificial anode, would prevent the iron metal from corroding. Sacrificial metals are widely used to prevent other metals from corroding: Because zinc is a more active metal than iron, it will act as the sacrificial anode in the. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From www.dreamstime.com
Sacrificial zinc anode stock photo. Image of reaction 308049266 Why Coating Of Zinc On Iron Is Called Sacrificial Anode Sacrificial coating is a type of metal coating which will undergo oxidation more than the metal surface that it protects. According to the table of standard reduction potentials,. Sacrificial metals are widely used to prevent other metals from corroding: Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. Magnesium has the most negative electropotential of the three.. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From kaide-anode.en.made-in-china.com
Marine Single Iron Leg Welding Zinc Alloy Sacrificial Anode China Why Coating Of Zinc On Iron Is Called Sacrificial Anode The addition of zinc, a sacrificial anode, would prevent the iron metal from corroding. Magnesium has the most negative electropotential of the three. [3] many steel objects are coated with a. A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged. Both zinc and aluminum produce potentials more. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From hsmarine.en.alibaba.com
Zinc Alloy Sacrificial Anode for Cathodic Protection System, View zinc Why Coating Of Zinc On Iron Is Called Sacrificial Anode Sacrificial coating is a type of metal coating which will undergo oxidation more than the metal surface that it protects. According to the table of standard reduction potentials,. Sacrificial protection of iron using zinc is explained through a diagram of the electrochemical processes involved. Magnesium has the most negative electropotential of the three. Sacrificial metals are widely used to prevent. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From www.slideserve.com
PPT USNA Chemistry Department PowerPoint Presentation, free download Why Coating Of Zinc On Iron Is Called Sacrificial Anode Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged. Sacrificial coating is a type of metal coating which will undergo oxidation more than the metal surface that it protects. Sacrificial anodes generally come in three. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From www.purehm.net
Sacrificial Anodes Why Coating Of Zinc On Iron Is Called Sacrificial Anode Magnesium has the most negative electropotential of the three. Sacrificial metals are widely used to prevent other metals from corroding: Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. Sacrificial protection of iron using zinc is explained through a diagram of the electrochemical processes involved. Because zinc is a more active metal than iron, it will act. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From stopcor.co.uk
Sacrificial Anodes Stopcor Why Coating Of Zinc On Iron Is Called Sacrificial Anode The addition of zinc, a sacrificial anode, would prevent the iron metal from corroding. For example in galvanised steel. Both zinc and aluminum produce potentials more negative. Sacrificial anodes generally come in three metals: According to the table of standard reduction potentials,. Sacrificial metals are widely used to prevent other metals from corroding: Magnesium has the most negative electropotential of. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From citimarinestore.com
When to Use Zinc Anodes or Aluminum Anodes on Your Boat Why Coating Of Zinc On Iron Is Called Sacrificial Anode The addition of zinc, a sacrificial anode, would prevent the iron metal from corroding. Sacrificial protection of iron using zinc is explained through a diagram of the electrochemical processes involved. According to the table of standard reduction potentials,. A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged.. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From korrosionsgruppen.se
Hull Anodes(Zinc Anodes) Cathodic Protection Sweden Why Coating Of Zinc On Iron Is Called Sacrificial Anode [3] many steel objects are coated with a. A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged. According to the table of standard reduction potentials,. Sacrificial coating is a type of metal coating which will undergo oxidation more than the metal surface that it protects. Sacrificial anodes. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From cathodicme.com
Zinc & Aluminium Sacrificial Anodes Systems Cathodic Marine Why Coating Of Zinc On Iron Is Called Sacrificial Anode For example in galvanised steel. Sacrificial anodes generally come in three metals: Because zinc is a more active metal than iron, it will act as the sacrificial anode in the electrochemical cell and dissolve (equation. Sacrificial metals are widely used to prevent other metals from corroding: Sacrificial protection of iron using zinc is explained through a diagram of the electrochemical. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From www.researchgate.net
Schematic showing cathodic protection methods using sacrificial anode Why Coating Of Zinc On Iron Is Called Sacrificial Anode The addition of zinc, a sacrificial anode, would prevent the iron metal from corroding. Sacrificial anodes generally come in three metals: Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. Both zinc and aluminum produce potentials more negative. Sacrificial metals are widely used to prevent other metals from corroding: For example in galvanised steel. A galvanic anode,. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From www.southernyachting.co.za
Sacrificial Anodes Southern Yachting Academy Why Coating Of Zinc On Iron Is Called Sacrificial Anode Sacrificial protection of iron using zinc is explained through a diagram of the electrochemical processes involved. According to the table of standard reduction potentials,. Both zinc and aluminum produce potentials more negative. Because zinc is a more active metal than iron, it will act as the sacrificial anode in the electrochemical cell and dissolve (equation. [3] many steel objects are. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From www.researchgate.net
Sacrificial anode system. Download Scientific Diagram Why Coating Of Zinc On Iron Is Called Sacrificial Anode Sacrificial coating is a type of metal coating which will undergo oxidation more than the metal surface that it protects. A galvanic anode, or sacrificial anode, is the main component of a galvanic cathodic protection system used to protect buried or submerged. Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. Both zinc and aluminum produce potentials. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From www.bbc.co.uk
BBC Two Bitesize Chemistry, Zinc as sacrificial protection to Why Coating Of Zinc On Iron Is Called Sacrificial Anode Magnesium has the most negative electropotential of the three. Sacrificial coating is a type of metal coating which will undergo oxidation more than the metal surface that it protects. According to the table of standard reduction potentials,. Because zinc is a more active metal than iron, it will act as the sacrificial anode in the electrochemical cell and dissolve (equation.. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From www.desperatesailors.com
How Do Sacrificial Anodes Work? Find Out Everything Here Why Coating Of Zinc On Iron Is Called Sacrificial Anode Common material choices for sacrificial anodes are zinc, aluminum, and their alloys. Because zinc is a more active metal than iron, it will act as the sacrificial anode in the electrochemical cell and dissolve (equation. For example in galvanised steel. Sacrificial coating is a type of metal coating which will undergo oxidation more than the metal surface that it protects.. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.
From www.nauticexpo.com
Ship sacrificial anode ERIS PROPELLERS zinc Why Coating Of Zinc On Iron Is Called Sacrificial Anode According to the table of standard reduction potentials,. Sacrificial anodes generally come in three metals: [3] many steel objects are coated with a. Because zinc is a more active metal than iron, it will act as the sacrificial anode in the electrochemical cell and dissolve (equation. For example in galvanised steel. Sacrificial coating is a type of metal coating which. Why Coating Of Zinc On Iron Is Called Sacrificial Anode.